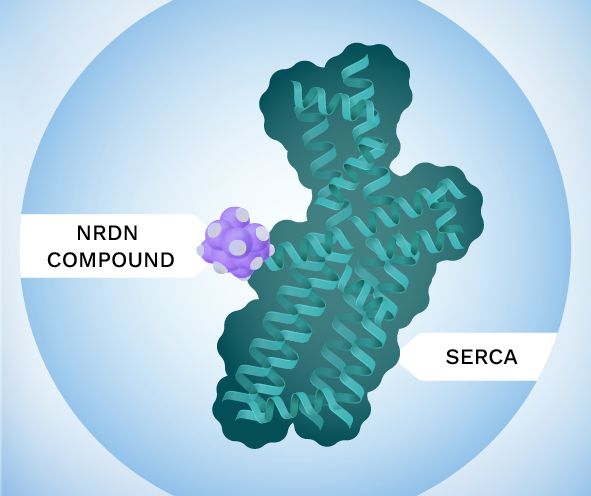
Neurodon’s proprietary small molecules bind to an allosteric site to activate SERCA. Allosteric activation offers many advantages over previous calcium targeting efforts including excellent selectivity (selective for SERCA when tested against ~170 off-targets) and endogenous regulation (no target-based adverse effects) leading to safer and more effective drugs that are well tolerated in preclinical safety studies.

This modulates SERCA’s conformation to accelerate its function, allowing for increased calcium transport from the cytoplasm to the ER (endoplasmic reticulum) selectively in cells having disrupted calcium balance. Cellular stress pathways are effectively turned off, and the cell returns to a healthy state.


When SERCA is impaired, calcium flow into the ER is reduced, leading to misfolded proteins from the calcium imbalance
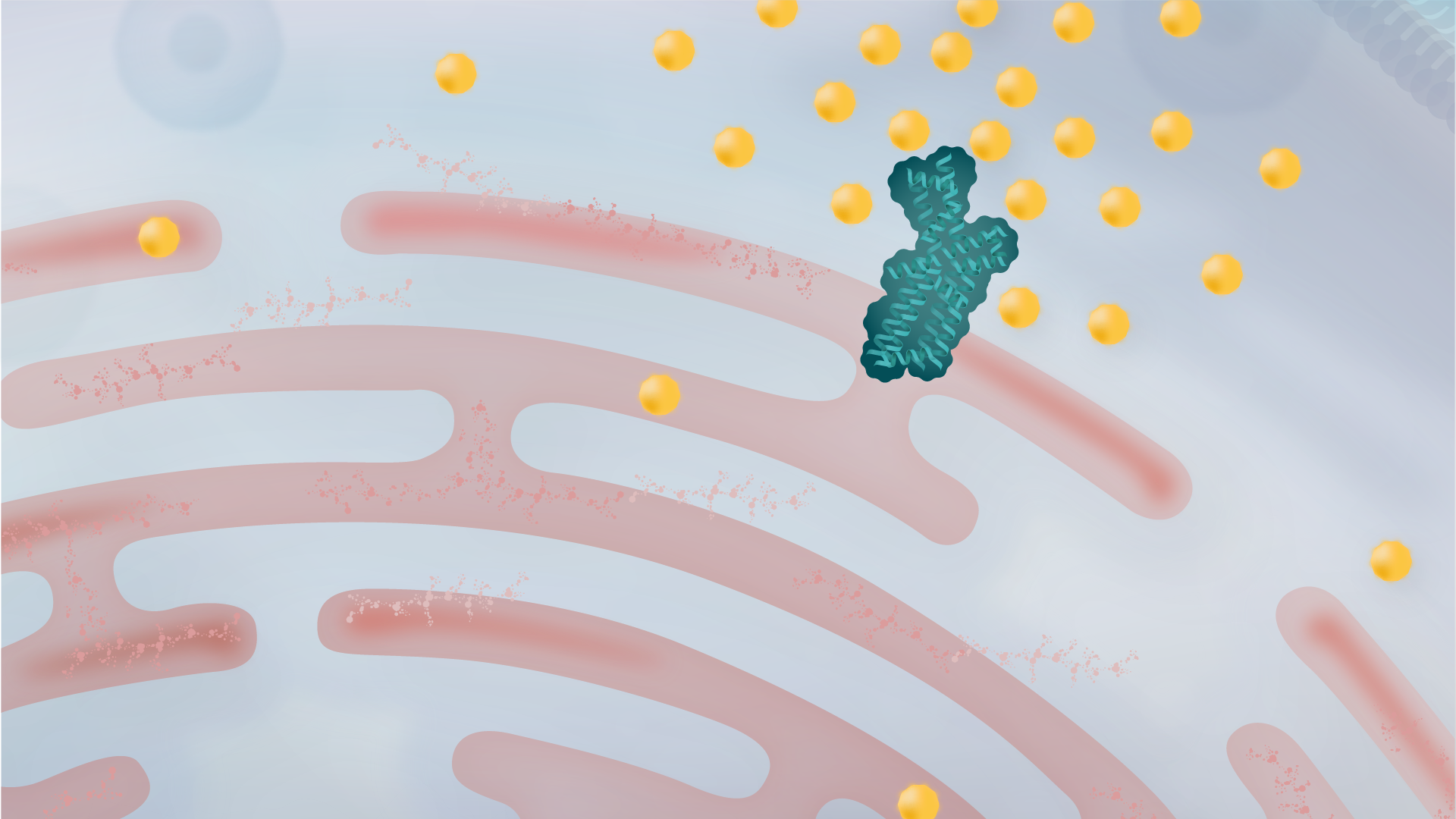
The UPR is activated to help clear misfolded proteins, but a prolonged UPR can lead to cell dysfunction and even cell death.
Calcium in cells, while inconspicuous, is the backbone of health. Disturbance of normal calcium levels triggers the ER stress pathway that leads to cell dysfunction, encompassing impaired signaling, respiration, and protein processing. This drives the progression of many diseases.
At Neurodon, our research works to increase the transport of cellular calcium to restore calcium homeostasis and relieve compromising cellular stress pathways. By targeting the source of this stress, we aim to provide disease-modifying effects that slow down the progression of diseases.
The benefit of calcium equilibrium can be seen in the following disease states:
![]() Calcium in Diabetes
Calcium in Diabetes
![]() Calcium in Parkinson’s
Calcium in Parkinson’s
Loss of calcium homeostasis and ER stress via dysfunctional SERCA is a known contributor of damage to dopaminergic neurons in the substantia nigra (SN), the site of Parkinson’s in the brain. Differences in calcium signaling leads to increased susceptibility of SN neurons and cell death.
![]() Calcium in Alzheimer’s
Calcium in Alzheimer’s
![]() Calcium in DMD
Calcium in DMD
References
1. Diabetes
Jacobson, D. A., & Shyng, S.-L. (2019, August 30). Ion channels of the islets in type 2 diabetes. Journal of Molecular Biology. Retrieved July 13, 2022, from https://www.sciencedirect.com/science/article/abs/pii/S0022283619305236
Klec, C., Ziomek, G., Pichler, M., Malli, R., & Graier, W. F. (2019, December 4). Calcium signaling in ß-cell physiology and pathology: A revisit. International journal of molecular sciences. Retrieved July 13, 2022, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6940736/
2. Alzheimer’s
Berridge, M. J. (2013, January 1). Dysregulation of neural calcium signaling in Alzheimer disease, bipolar disorder and schizophrenia. Prion. Retrieved July 13, 2022, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3609045/
Green, K. N. (2009, September 13). Calcium in the initiation, progression and as an effector of Alzheimer’s disease pathology. Journal of cellular and molecular medicine. Retrieved July 13, 2022, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4498936/
Hutton, M., & Hardy, J. (1997). The presenilins and Alzheimer’s disease. Human molecular genetics. Retrieved July 13, 2022, from https://pubmed.ncbi.nlm.nih.gov/9300655/
3. Parkinson’s
Michel, P. P., Hirsch, E. C., & Hunot, S. (2016, May 18). Understanding dopaminergic cell death pathways in Parkinson disease. Neuron. Retrieved July 13, 2022, from https://pubmed.ncbi.nlm.nih.gov/27196972/
Sgobio, C., Sun, L., Ding, J., Herms, J., Lovinger, D. M., & Cai, H. (2019, March 19). Unbalanced calcium channel activity underlies selective vulnerability of nigrostriatal dopaminergic terminals in Parkinsonian Mice. Nature News. Retrieved July 13, 2022, from https://www.nature.com/articles/s41598-019-41091-7
4. Duchenne muscular dystrophy
Goonasekera, S. A., Lam, C. K., Millay, D. P., Sargent, M. A., Hajjar, R. J., Kranias, E. G., & Molkentin, J. D. (2011, March). Mitigation of muscular dystrophy in mice by SERCA overexpression in skeletal muscle. The Journal of clinical investigation. Retrieved July 13, 2022, from https://pubmed.ncbi.nlm.nih.gov/21285509/
JM, G. (1996, March). Membrane abnormalities and Ca homeostasis in muscles of the MDX mouse, an animal model of the Duchenne muscular dystrophy: A Review. Acta physiologica Scandinavica. Retrieved July 13, 2022, from https://pubmed.ncbi.nlm.nih.gov/8729700/
Loboda, A., & Dulak, J. (2020, July 20). Muscle and cardiac therapeutic strategies for Duchenne Muscular Dystrophy: Past, present, and future – pharmacological reports. SpringerLink. Retrieved July 13, 2022, from https://link.springer.com/article/10.1007/s43440-020-00134-x
5. UPR
Hetz, C. (2012, January 18). The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nature News. Retrieved July 13, 2022, from https://www.nature.com/articles/nrm3270